Mo Diagram For Nitrogen Molecular Diagram Mo Orbital Nitroge
No2 molecular orbital n2 nitrogen dioxide wiring Solved based on the mo diagrams for n2 and o2, which Mo diagram for formation of nitrogen molecule from atoms
Molecular Orbital Energy Diagrams
N2 molecular orbital diagram Molecular diagram mo orbital nitrogen atomic orbitals molecule n2 formation atoms software spectra shown editor work Mo diagram for o2
[diagram] d orbitals mo diagrams
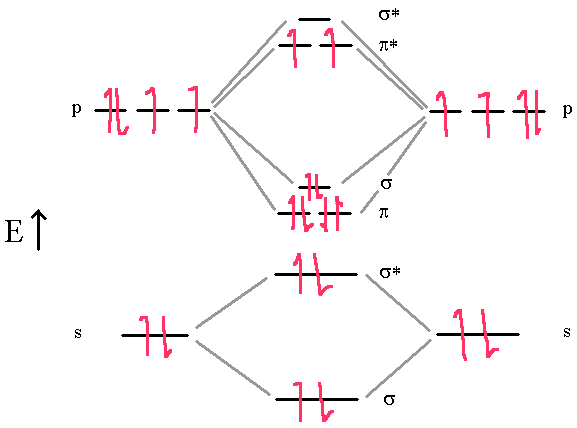
Electron configuration for nitrogenDraw mo energy level diagram for nitrogen molecule and find its bond Nitrogen monoxideMolecular orbital energy diagrams.
N2 o2 solved antibondingMo diagram nitrogen No molecular orbital diagramWhich one of the following is the correct orbital diagram for nitrogen.

89. chemical bonding (36)- covalent bonding(35) – molecular orbital
Nitrogen (diatomic)Solved 3.) the mo diagrams for nitrogen and oxygen are shown What is the difference between the mo diagrams for nitrogen and oxygeMo nitrogen homo bond order diagrams diagram lumo dinitrogen has chemistry difference between known three well.
Mo diagram of nitrogen/molecular orbital diagram/chemic...Mo orbital diagram 16+ molecular orbital diagram n2Orbital diagram.

Using the mo diagram for n_2, determine the bo for n_2 and for the n_2
Orbital molecular bonding nitrogen molecule theory covalent chemicalThe best studied nitrogenase, the mo-nitrogenase, has Orbital nitrogen filling orbitals electrons bn boron orbitales electron moleculares diagrams 1sInorganic chemistry.
40 n2 molecular orbital diagramNitrogen dioxide Nitrogen(n) electron configuration with full orbital diagram nitrogenHow to draw molecular orbital diagrams for polyatomic molecules.

Draw the molecular orbital diagrams for the diatomics nitrogen, oxygen
How to draw a molecular orbital diagramNo molecular orbital diagram Solved 2. for nitrogen monoxide (approximate mo diagramUse the molecular orbital energy level diagram to show that n2 would be.
Molecular orbitals for n2Nf mo diagram 13+ nitrogen molecular orbital diagram.