Molecular Orbital Diagrams Explained No Molecular Orbital Di
A simplified guide to understanding molecular orbital diagrams Orbital electron orbitals atoms chemistry dimensional depicted Molecular orbital theory (made easy)
1.2. The molecular orbital diagrams of a) O 2 − doublet (n = 1
Top notch tips about how to draw orbital diagrams 37+ molecular orbital geometry image Orbital sulfur monahan caroline
Molecular orbital theory
Electron orbitals electrons quantum numbers chemistry atoms structure electronic introductory model orbital figure atomic energy number arrangement ball libretexts chapterOrbitals orbital molecular bonding chemistry localized hybridization geometry sp atoms highland involving chem libretexts formation A simplified guide to understanding molecular orbital diagramsOrbital orbitals two atomic axis internuclear chem covalent chemistry libretexts combining creates sigma pageindex.
1.2. the molecular orbital diagrams of a) o 2 − doublet (n = 112+ atomic orbital diagram [9+] full color molecular orbital diagram and the descriptionIntroductory chemistry 1.0.

Molecular orbital diagram oxygen
Use the appropriate molecular orbital energy diagram to write theOrbital diagrams — overview & examples 11.5: molecular orbital theoryMolecular orbital (mo) diagram of 5d-orbitals transition-metal oxides.
No molecular orbital diagramOrbital theory molecular ppt mo atomic powerpoint orbitals presentation diatomic molecules 1.1. molecular orbital diagram of o 2 in the ground triplet state, withMolecular orbital diagram explained.
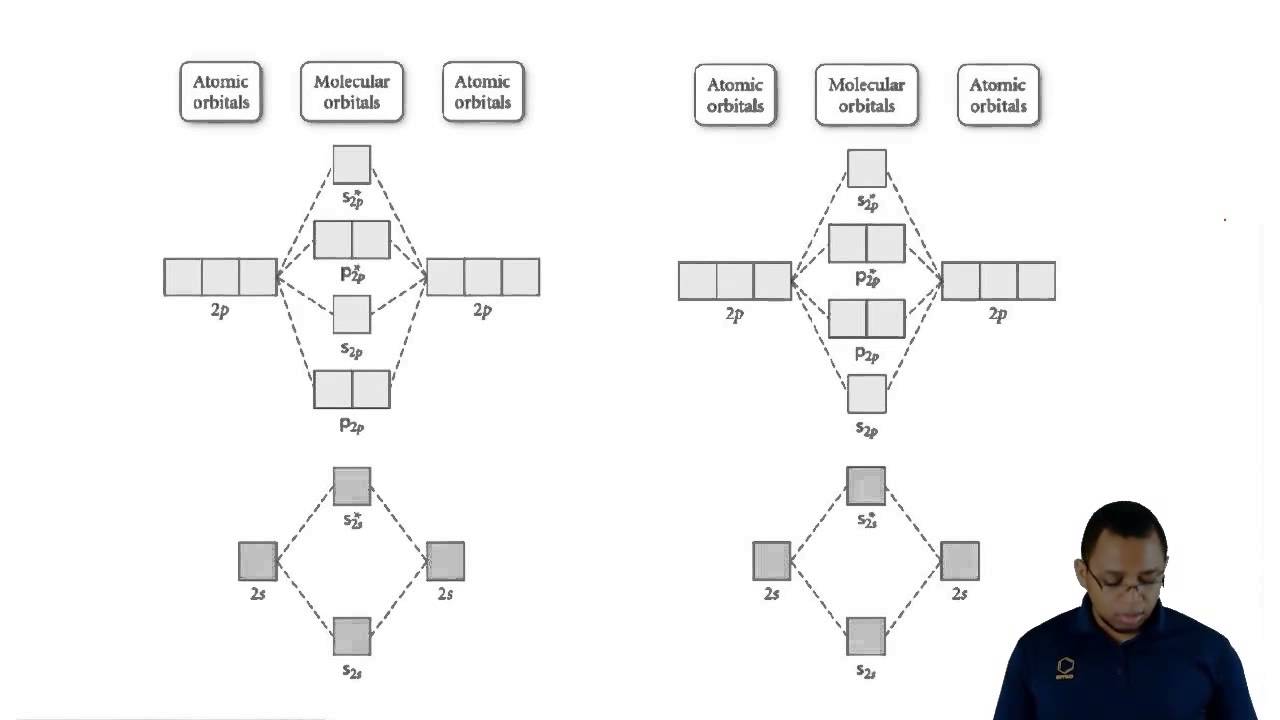
Orbital molecular o2 ozone oxygen paramagnetic molecule bonding libretexts orbitals valence molecules chem dissociation
38 o2 2- molecular orbital diagramNo molecular orbital diagram Molecular orbital diagram for nitrogen monoxide, the nitrosyl cationMolecular orbital orbitals sulfur bonding delocalized valence electrons libretexts atomic atom contributes.
Draw the molecular orbital diagram of $\ce{be2-}$.Understanding molecular orbital theory N2o molecular orbital diagramOrbital molecules theory orbitals diatomic of2 bonding chemistry delocalized diagrams chem homonuclear libretexts electrons hybridization geometry correlation valence equal atoms.

No molecular orbital diagram
Orbital nitrogen molecular orbitals electrons boron orbitales electron molecularesThe molecular orbital diagram of no shown in figure Atomic orbitals explainedA simplified guide to understanding molecular orbital diagrams.
Chapter 5.3 delocalized bonding and molecular orbitalsNo molecular orbital diagram Molecular ozone diagram bonding orbital orbitals antibonding mo bonds theory molecule nonbonding electrons delocalized polyatomic multiple chemistry resonance example benzenePolyatomic systems with multiple bonds.

8.4: molecular orbital theory
Molecular orbital diagram of bn .
.







