Molecular Orbital Energy Diagram Orbital Molecular Diagram C
Day 6: molecular orbitals; lewis structures – chemistry 109, fall 2020 What is the molecular orbital energy diagram for $\ce{li2}$? 1.11: molecular orbital theory
Molecular Orbitals | Introductory Chemistry
Energy level diagram for molecular orbitals What are antibonding molecular orbitals? + example Use the appropriate molecular orbital energy diagram to write the
Molecular orbitals orbital diagram energy diatomic molecules atomic homonuclear atoms figure number made electron config
1.1. molecular orbital diagram of o 2 in the ground triplet state, withMolecular orbital molecule orbitals wisc unizin Energy level diagram for molecular orbitalsOrbital molecular diagram oxide orbitals nitric diatomic energy level mo molecule theory principles molecules electron valence electrons chemistry bonding general.
16+ molecular orbital diagram n2Draw the molecular orbital energy level diagram of toppr com Understanding molecular orbital theoryOrbital o2 oxygen theory paramagnetic ozone diagrams molecule bonding valence molecules libretexts orbitals electrons.

Chemical bonding
Molecular orbitals – introductory chemistry- 1st canadian editionO2 molecular orbital diagram Molecular orbital orbitals n2 bonding o2 ionMolecular orbital diagrams simplified – megan lim – medium.
Energy molecular orbital diagram level f2 n2 o2 ne2 chemistry orbitals bonding chemical classOrbital energy diagram Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level cl2 libretexts electron second delocalized homonuclear row20+ n2+ molecular orbital diagram.

Molecular orbital diagram energy molecules diatomic atomic homonuclear orbitals atoms heteronuclear chemistry figure number made 2p
Electron orbitals electrons quantum numbers chemistry atoms structure electronic introductory model orbital figure atomic energy number arrangement ball libretexts chapterNo molecular orbital diagram Orbital molecular diagram cl2 s2 molecule orbitals electron bond bonding c2 energy theory valence unpaired sulfur chlorine paramagnetic electrons according10.5: molecular orbital theory.
Diagram orbital molecular ozone bonding orbitals antibonding mo theory molecule electrons bonds delocalized chemistry resonance example multiple polyatomic pi bondOrbital molecular diagrams heteronuclear diatomic molecules diagram simplified atoms medium two Molecular orbitalsIntroductory chemistry 1.0.
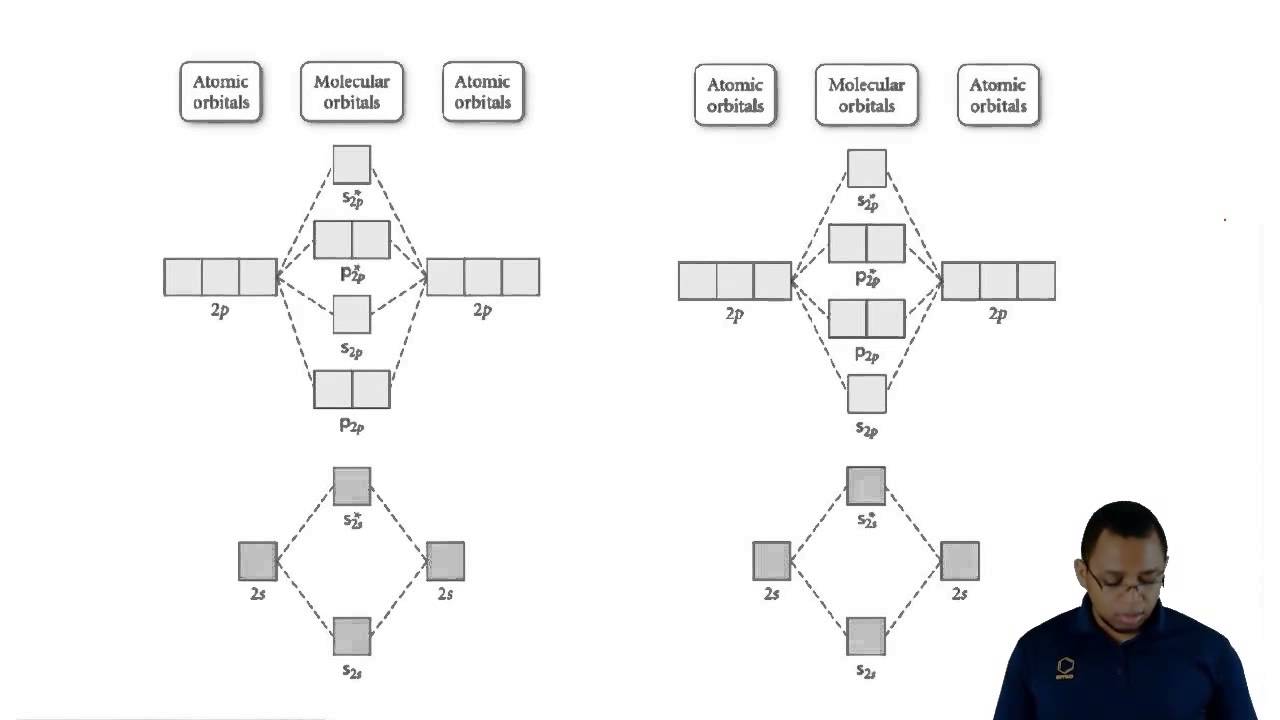
Molecular orbitals determination
9.10: molecular orbital theory predicts that molecular oxygen isEnergy molecular level diagram orbitals orbital n2 o2 bonding chemistry structure order f2 chemical hi ne2 No molecular orbital diagramMolecular orbitals.
Molecular diagram energy orbital hydrogen electron configuration orbitals chemistry h2 mo introductory figureMolecular orbital energy level diagrams -hydrogen, hypothetical Molecular orbital theoryOrbital energy molecular theory diagram orbitals mo two atomic rules principle these first stable.

Mo theory
[9+] full color molecular orbital diagram and the descriptionMolecular orbital theory (made easy) No molecular orbital diagram11.5: molecular orbital theory.
40 complete the mo energy diagram for the n2+ ion.Use the molecular orbital energy level diagram to show that n2 would be [diagram] pi bond molecular orbital diagram.








